Henry's law
The principle of our vacuum degassers is based on Henry's law!
The state of the water (pressure and temperature) in a vacuum degasser can be projected in a graph that illustrates a heating system following Henry’s law. The vacuum degasser removes all gases from the system through continuously returning water to it that has extremely low concentrations of gases. Water with the lowest concentrations of dissolved gases is found in the vacuum degasser.
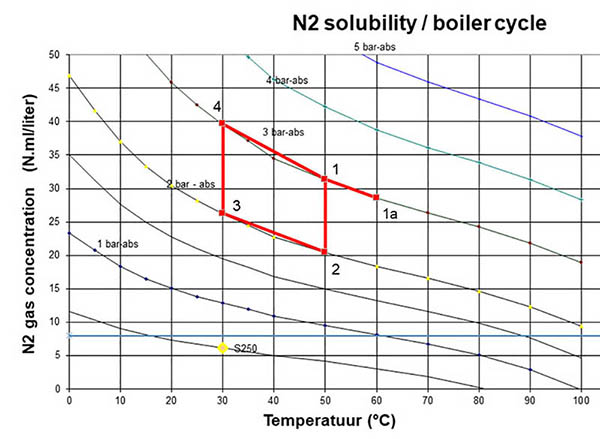
As shown in this example of the Spirotech Superior S250: a boiler in the basement operates at a low temperature (50-30°C). With a static height of 10 metres. According to the system projected in the graph, you can see the boiler is fed 30°C at 2 bar in the return (4), 50°C at 2 bar in the flow (1), boiler wall 60°C (1a). Traditionally, this is the point at which gases were released.
After point 1 the water flows upwards in the system (this is downwards in the graph as the pressure is reduced) to the top floor energy consumer (e.g. radiator, underfloor heating) at 50°C and 1 bar (2). Here the system water to 30°C while pressure is at 1 bar (3). The water flows back down inside the building, increasing the pressure as it returns to the boiler at 30°C and 2 bar.
All these points have specific maximum dissolved gas concentrations. We can use this data to predict the best possible place for removing gases, particularly because the point with the lowest gas concentration is the point where the most released gases in the form of free air or microbubbles are available.
If we separate the microbubbles here, the entire system will be free of air, as this ensures that the actual gas concentration is kept lower than the maximum gas concentration at any given point in the system. It also means that all air in the system will be absorbed by the water that is “hungry”, as it has been treated to keep it below its maximum gas concentration.
With an vacuum degasser installed, the release of gases is regulated, so none escape from other places in the system.
If we return to the graph illustrating the system, we see the lowest gas concentration is 20.5 Nml/l. This is to be found at the highest point in the system. However, there can be several high points, making it impossible to predict the best point for releasing the gases.
For example, incorporating an SpiroVent Superior S250 in the system’s return to the boiler (marked by the yellow dot) will result in water being returned to the system with extremely low gas saturation (6 Nml/l). Returning this water to the system will reduce the concentration of gas in the system water to such a degree that it will absorb any gas it encounters anywhere in the system. This includes air pockets in underfloor heating and in the tops of radiators. With an SpiroVent Superior installed, system water will be undersaturated, ensuring a trouble free operation of the system.
